Alpharadin wlll be new treatment option for Prostate Cancer
One piece of hot news at the 2011 European Multidisciplinary Cancer Congress (twitter #EMCC2011) taking place in Stockholm this weekend is the data on radium-223 chloride (Alpharadin) in metastatic castration resistant prostate cancer. The phase 3 ALSYMPCA trial results were presented in yesterday’s presidential symposia by Dr Chris Parker, Consultant Clinical Oncologist at The Royal Marsden Hospital.

The Scandinavian location for the presentation could not have been better, given that Alpharadin was developed by the Norwegian company Algeta. Bayer Schering Pharma AG have the worldwide commercial rights, but Algeta maintains a co-promotion option in the United States.
I first picked up on Alpharadin in a presentation given at the American Urological Association (AUA) annual meeting by Oliver Sartor (Tulane) earlier this year when he reviewed new prostate cancer products in development.
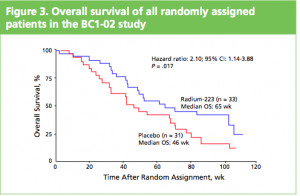 At the ASCO 2011 meeting in Chicago there was a poster on the Alpharadin Phase 2 trial data (see the figure on the right) that caught my attention given that it showed an overall survival (OS) advantage. This news was, however, largely drowned by the interest in cabozantinib (XL184).
At the ASCO 2011 meeting in Chicago there was a poster on the Alpharadin Phase 2 trial data (see the figure on the right) that caught my attention given that it showed an overall survival (OS) advantage. This news was, however, largely drowned by the interest in cabozantinib (XL184).
The result is that Alpharadin has to many come out of left field. It is a promising compound for the treatment of prostate cancer that will provide new treatment options for patients with metastatic disease. In particular, use in combination with other therapies such as abiraterone acetate (Zytiga) may prolong survival to a greater extent than either does individually.
Currently, radium-223 chloride (Alpharadin) is only in investigational use and is not approved in Europe or the United States. It is, however, on the fast track towards FDA approval in 2012.
 What makes Alpharadin exciting as a new treatment option for castration resistant prostate cancer (CRPC) is that the ALSYMPCA trial data shows that it not only provides a significant median overall survival (OS) benefit of 2.8 months compared to placebo (14 months versus 11.2 months, p=0.00185, HR 0.695), but significantly delays the time to first skeletal event by 5.2 months (13.6 months versus 8.4 months, p=0.00046, HR 0.610).
What makes Alpharadin exciting as a new treatment option for castration resistant prostate cancer (CRPC) is that the ALSYMPCA trial data shows that it not only provides a significant median overall survival (OS) benefit of 2.8 months compared to placebo (14 months versus 11.2 months, p=0.00185, HR 0.695), but significantly delays the time to first skeletal event by 5.2 months (13.6 months versus 8.4 months, p=0.00046, HR 0.610).
The overal survival (OS) benefit seen in the ALYSMPCA phase 3 trial is comparable to other approved agents in the post-docetaxel setting for CRPC. However, where it is unique is in the additional effect it has on skeletal related events (SRE), a common occurrence in metastatic prostate cancer. Bone metastases are painful and have a significant impact on quality of life.
Other compounds that target the bone microenviroment such as denosumab (Xgeva), provide a delay in the time to first skeletal event in prostate cancer patients but to-date have not been shown to confer an overall survival advantage. This means that Alphardin is the first bone targeted agent to confer both an overall survival and a delay in time to first skeletal event.
After Dr Parker’s presentation of the ALSYMPCA phase 3 trial data yesterday here in Stockholm, Professor Wim Oyen of the Department of Nuclear Medicine in Nijmegen discussed the data.
What he noted was the high tolerability of Ra-223 chloride (Alpharadin) as compared to other radiopharmaceuticals for treatment of patients with bone metastases. He discussed how the emission of alpha particles allows for a short range effect (a few cell diameters) that is very localized, but with a large biological effect.
Oyen highlighted the “opportunity for improving patient outcome by adding Ra-223 in regimens of combination therapy,” something that Dr Parker speculated about in his media briefing.
Professor Oyen also saw “an opportunity for improving patient outcome by using Ra-223 in an adjuvant setting.” His conclusion based on the phase 3 ALSYMPCA trial data presented was that radium-223 chloride (Alpharadin) is an “effective, very well tolerated and convenient treatment modality.”
Dr Parker mentioned to me, while waiting for a train back to Stockholm, that the ALSYMPCA trial data he presented had not yet been submitted for publication. He said he would be disappointed if it did not appear in the New England Journal of Medicine. Given that it is groundbreaking and “practice changing,” I would be surprised if it is not published in the NEJM in due course.
I am sure that we will be hearing more about radium-223 chloride (Alpharadin) in the forthcoming months, especially now it is on fast track to FDA approval in 2012.
Although not a cure for prostate cancer, the ALSYMPCA trial data presented here in Stockholm is further good news for patients, and will provide a potential new treatment option for urologists and oncologists.
One Response to “Alpharadin wlll be new treatment option for Prostate Cancer”
[…] Post navigation ← Previous […]
Comments are closed.